Rat Stem Cells Restore Mouse Brain Circuits
Studies demonstrate the regeneration of mouse brain circuits with rat stem cells, providing new insights into neurological restoration and cross-species brain development. Credit: SciTechDaily.com
Research teams have successfully regenerated mouse brain circuits using rat stem cells, showcasing a new method for restoring brain function and studying interspecies brain development.
These findings open up possibilities for treating neurological diseases and understanding brain evolution, while also hinting at future clinical applications and ethical challenges in using similar techniques for human organ transplantation.
Scientists Regenerate Neural Pathways in Mice With Cells From Rats
Two independent research groups have successfully restored brain circuits in mice using neurons derived from rat stem cells. Recently published in the journal Cell, these studies provide important insights into brain tissue development and open up new possibilities for rejuvenating brain functions lost to diseases and aging.
“This research helps to show the brain’s potential flexibility in using synthetic neural circuits to restore brain functions,” says Kristin Baldwin, a professor at Columbia University in New York and corresponding author of one of the two papers. Baldwin’s team restored mouse olfactory neural circuits, the interconnected neurons in the brain responsible for the sense of smell, and their function using stem cells from rats.
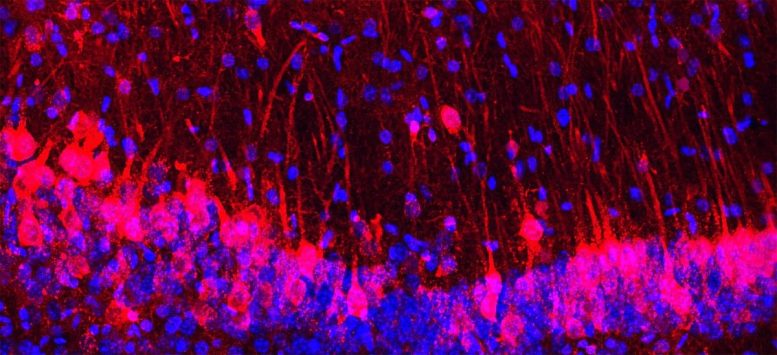
Mouse hippocampus with rat cells (red) and nuclei of both mouse and rat cells (blue). Credit: M. Khadeesh Imtiaz, Columbia University Irving Medical Center
Interspecies Genetic Engineering and Its Implications
“Being able to generate brain tissues from one species inside another can help us understand brain development and evolution in different species,” says Jun Wu, an associate professor at the University of Texas Southwestern Medical Center in Dallas and corresponding author of the other paper. Wu’s team developed a CRISPR-based platform that could efficiently identify specific genes that drive the development of specific tissues. They tested the platform by silencing a gene needed for forebrain development in mice and then restoring the tissue using rat stem cells.
Mice and rats are two distinct species that evolved independently for approximately 20 to 30 million years. In previous experiments, scientists were able to replace pancreases in mice using rat stem cells through a process called blastocyst complementation. For this process to work, researchers inject rat stem cells into mice blastocysts—early-stage embryos—that lack the ability to develop a pancreas due to genetic mutations. The rat stem cells then developed into the missing pancreas and complemented its function.
Breakthroughs in Brain Tissue Regeneration
But, to date, generating brain tissues using stem cells from a different species through blastocyst complementation has not been reported. Now, using CRISPR, Wu’s team tested seven different genes and found that knocking out Hesx1 could reliably generate mice that had no forebrain. The team then injected rat stem cells in blastocysts of Hesx1 knockout mice, and the rat cells filled in the niche to form a forebrain in mice. Rats have bigger brains than mice, but the rat-origin forebrains developed at the same pace and size as that of mice. In addition, rat neurons were able to transmit signals to the neighboring mouse neurons and vice versa.
The researchers didn’t test whether the forebrain from rat stem cells changed mice’s behaviors. “There’s a lack of good behavioral tests to distinguish rats from mice,” Wu says. “But from our experiment, it seems like these mice with rat forebrain don’t behave out of the ordinary.”
Advanced Applications and Future Prospects
In the other study, Baldwin’s team used specific genes to either kill or silence mouse olfactory sensory neurons used for the sense of smell and injected rat stem cells into the mice embryos. The silencing model mimics what is seen in neurodevelopmental disorders, where certain neurons cannot communicate well with the brain. The killing model removed the neurons entirely, simulating degenerative diseases.
They found blastocyst complementation restored mouse olfactory neural circuits differently depending on the model. When mouse neurons were present but silent, the rat neurons helped form better-organized brain regions compared to the killing model. However, when the team tested these rat-mouse chimeras by training them to find a hidden cookie buried in a cage, rat neurons were best at rescuing behaviors in the killing model.
“This really surprising result allows us to look at what’s different between those two disease models and try to identify mechanisms that could help restore functions in either type of brain disease,” Baldwin says. Her team also tested blastocyst complementation in disease model mice using cells from mice with normal olfactory systems. They showed that intraspecies complementation rescued cookie finding in both models.
Exploring the Frontiers of Medical Science
“Right now, people are being transplanted with stem cell-derived neurons for Parkinson’s disease and epilepsy in clinical trials. How well will that work? And will different genetic backgrounds between the patient and the transplanted cells pose a barrier? This study provides a system in which we can evaluate the possibilities for same species brain complementation at a much larger scale than a clinical trial,” Baldwin says.
Blastocyst complementation is still far from clinical application in humans, but both studies suggest stem cells from different species can synchronize their development with the host’s brain.
Scientists have also been experimenting with growing human organs in other species like pigs using blastocyst complementation. Last year, scientists generated embryonic kidneys using human stem cells in pigs, offering a potential solution for the many people on waitlists for transplants.
“Our aspiration is to enrich pig organs with a certain percentage of human cells, with the aim of improving outcomes for organ recipients. But currently, there are still many technical and ethical challenges that we need to overcome before we can test this in clinical trials,” says Wu.
Besides the studies’ implications in medicine, the teams are also interested in using this approach to study the brains of many wild rodents that were not accessible in the laboratory setting.
“There are over 2,000 living rodent species in the world. Many of them behave differently from the rodents we commonly study in the lab. Interspecies neural blastocyst complementation can potentially open the door to study how the brains from those species develop, evolve, and function,” Wu says.
For more on this research, see Mice Engineered With Rat Neurons Show Advanced Sensory Skills.
References:
“Functional sensory circuits built from neurons of two species” by Benjamin T. Throesch, Muhammad Khadeesh bin Imtiaz, Rodrigo Muñoz-Castañeda, Masahiro Sakurai, Andrea L. Hartzell, Kiely N. James, Alberto R. Rodriguez, Greg Martin, Giordano Lippi, Sergey Kupriyanov, Zhuhao Wu, Pavel Osten, Juan Carlos Izpisua Belmonte, Jun Wu and Kristin K. Baldwin, 25 April 2024, Cell.
DOI: 10.1016/j.cell.2024.03.042
“Generation of rat forebrain tissues in mice” by Jia Huang, Bingbing He, Xiali Yang, Xin Long, Yinghui Wei, Leijie Li, Min Tang, Yanxia Gao, Yuan Fang, Wenqin Ying, Zikang Wang, Chao Li, Yingsi Zhou, Shuaishuai Li, Linyu Shi, Seungwon Choi, Haibo Zhou, Fan Guo, Hui Yang and Jun Wu, 25 April 2024, Cell.
DOI: 10.1016/j.cell.2024.03.017