A New Era in Non-Invasive Brain Healing
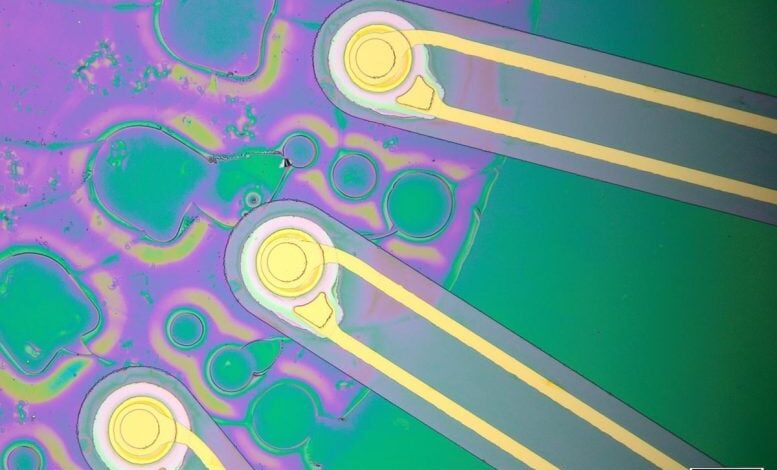
The ImPULS device contains ultrasound transducers and electrodes (gold) encapsulated within a polymer. Credit: Courtesy of the researchers
MIT’s implantable ImPULS device could become an alternative to the electrodes now used to treat Parkinson’s and other diseases.
MIT engineers developed a hair-thin ultrasound device that offers a potential breakthrough in treating neurological disorders by providing precise, minimally invasive deep brain stimulation. This technology, known as ImPULS, could replace traditional electrode-based methods, promising reduced tissue damage and increased efficacy.
Deep brain stimulation, by implanted electrodes that deliver electrical pulses to the brain, is often used to treat Parkinson’s disease and other neurological disorders. However, the electrodes used for this treatment can eventually corrode and accumulate scar tissue, requiring them to be removed.
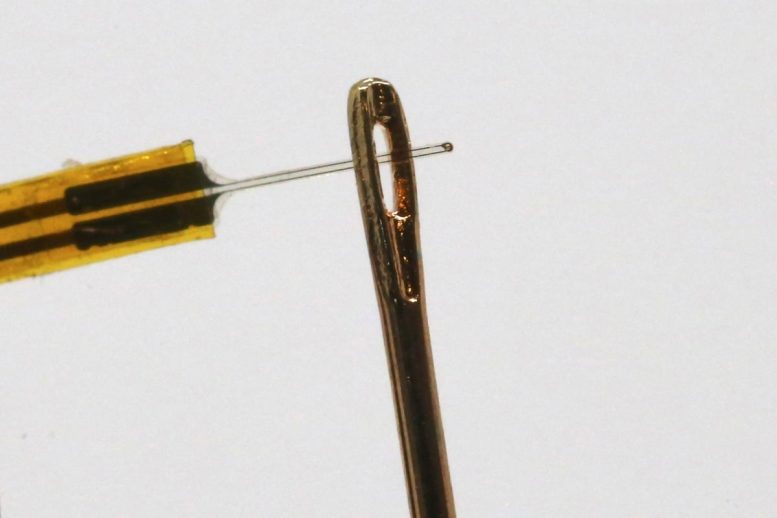
MIT researchers have now developed an alternative approach that uses ultrasound instead of electricity to perform deep brain stimulation, delivered by a fiber about the thickness of a human hair. In a study of mice, they showed that this stimulation can trigger neurons to release dopamine, in a part of the brain that is often targeted in patients with Parkinson’s disease.
“By using ultrasonography, we can create a new way of stimulating neurons to fire in the deep brain,” says Canan Dagdeviren, an associate professor in the MIT Media Lab and the senior author of the new study. “This device is thinner than a hair fiber, so there will be negligible tissue damage, and it is easy for us to navigate this device in the deep brain.”
Advantages of Ultrasound-Based Stimulation
In addition to offering a potentially safer way to deliver deep brain stimulation, this approach could also become a valuable tool for researchers seeking to learn more about how the brain works.
MIT graduate student Jason Hou and MIT postdoc Md Osman Goni Nayeem are the lead authors of the paper, along with collaborators from MIT’s McGovern Institute for Brain Research, Boston University, and Caltech. The study was published on June 4 in the journal Nature Communications.
Deep in the Brain
Dagdeviren’s lab has previously developed wearable ultrasound devices that can be used to deliver drugs through the skin or perform diagnostic imaging on various organs. However, ultrasound cannot penetrate deeply into the brain from a device attached to the head or skull.
“If we want to go into the deep brain, then it cannot be just wearable or attachable anymore. It has to be implantable,” Dagdeviren says. “We carefully customize the device so that it will be minimally invasive and avoid major blood vessels in the deep brain.”
Deep brain stimulation with electrical impulses is FDA-approved to treat symptoms of Parkinson’s disease. This approach uses millimeter-thick electrodes to activate dopamine-producing cells in a brain region called the substantia nigra. However, once implanted in the brain, the devices eventually begin to corrode, and scar tissue that builds up surrounding the implant can interfere with the electrical impulses.
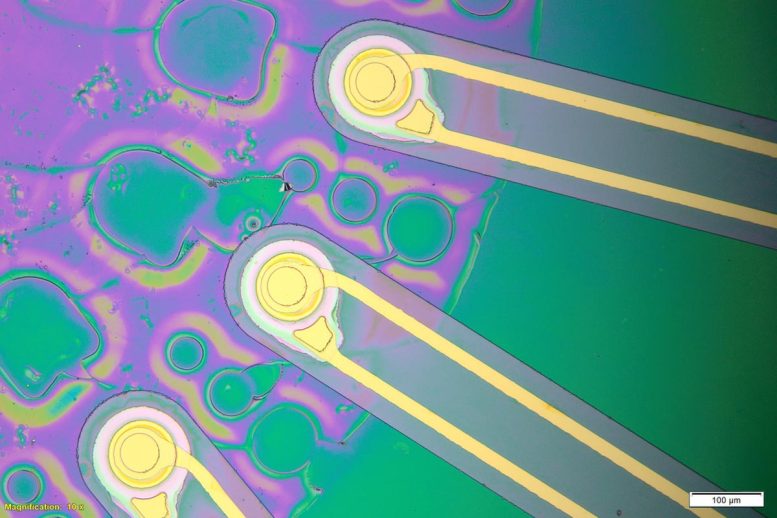
The new approach uses ultrasound delivered by a fiber about the thickness of a human hair. Credit: Courtesy of the researchers
New Design and Application of Ultrasound in the Brain
The MIT team set out to see if they could overcome some of those drawbacks by replacing electrical stimulation with ultrasound. Most neurons have ion channels that are responsive to mechanical stimulation, such as the vibrations from sound waves, so ultrasound can be used to elicit activity in those cells. However, existing technologies for delivering ultrasound to the brain through the skull can’t reach deep into the brain with high precision because the skull itself can interfere with the ultrasound waves and cause off-target stimulation.
“To precisely modulate neurons, we must go deeper, leading us to design a new kind of ultrasound-based implant that produces localized ultrasound fields,” Nayeem says. To safely reach those deep brain regions, the researchers designed a hair-thin fiber made from a flexible polymer. The tip of the fiber contains a drum-like ultrasound transducer with a vibrating membrane. When this membrane, which encapsulates a thin piezoelectric film, is driven by a small electrical voltage, it generates ultrasonic waves that can be detected by nearby cells.
“It’s tissue-safe, there’s no exposed electrode surface, and it’s very low-power, which bodes well for translation to patient use,” Hou says.
Implications and Future Directions
In tests in mice, the researchers showed that this ultrasound device, which they call ImPULS (Implantable Piezoelectric Ultrasound Stimulator), can provoke activity in neurons of the hippocampus. Then, they implanted the fibers into the dopamine-producing substantia nigra and showed that they could stimulate neurons in the dorsal striatum to produce dopamine.
“Brain stimulation has been one of the most effective, yet least understood, methods used to restore health to the brain. ImPULS gives us the ability to stimulate brain cells with exquisite spatial-temporal resolution and in a manner that doesn’t produce the kind of damage or inflammation as other methods. Seeing its effectiveness in areas like the hippocampus opened an entirely new way for us to deliver precise stimulation to targeted circuits in the brain,” says Steve Ramirez, an assistant professor of psychological and brain sciences at Boston University, and a faculty member at B.U.’s Center for Systems Neuroscience, who is also an author of the study.
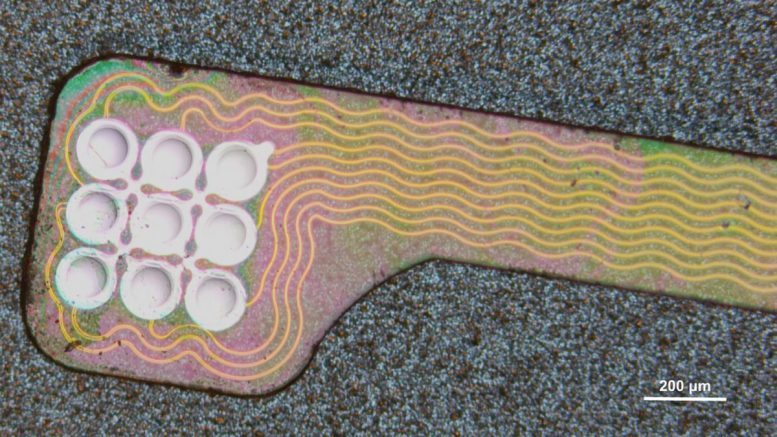
In the new system, transducers (silver) are powered by wires (gold) that deliver electrical stimulation. Credit: Courtesy of the researchers
Customizable and Scalable Technology
All of the components of the device are biocompatible, including the piezoelectric layer, which is made of a novel ceramic called potassium sodium niobate, or KNN. The current version of the implant is powered by an external power source, but the researchers envision that future versions could be powered a small implantable battery and electronics unit.
The researchers developed a microfabrication process that enables them to easily alter the length and thickness of the fiber, as well as the frequency of the sound waves produced by the piezoelectric transducer. This could allow the devices to be customized for different brain regions.
“We cannot say that the device will give the same effect on every region in the brain, but we can easily and very confidently say that the technology is scalable, and not only for mice. We can also make it bigger for eventual use in humans,” Dagdeviren says.
Conclusion and Future Research Goals
The researchers now plan to investigate how ultrasound stimulation might affect different regions of the brain, and if the devices can remain functional when implanted for year-long timescales. They are also interested in the possibility of incorporating a microfluidic channel, which could allow the device to deliver drugs as well as ultrasound.
In addition to holding promise as a potential therapeutic for Parkinson’s or other diseases, this type of ultrasound device could also be a valuable tool to help researchers learn more about the brain, the researchers say.
“Our goal to provide this as a research tool for the neuroscience community, because we believe that we don’t have enough effective tools to understand the brain,” Dagdeviren says. “As device engineers, we are trying to provide new tools so that we can learn more about different regions of the brain.”
Reference: “An implantable piezoelectric ultrasound stimulator (ImPULS) for deep brain activation” by Jason F. Hou, Md Osman Goni Nayeem, Kian A. Caplan, Evan A. Ruesch, Albit Caban-Murillo, Ernesto Criado-Hidalgo, Sarah B. Ornellas, Brandon Williams, Ayeilla A. Pearce, Huseyin E. Dagdeviren, Michelle Surets, John A. White, Mikhail G. Shapiro, Fan Wang, Steve Ramirez and Canan Dagdeviren, 4 June 2024, Nature Communications.
DOI: 10.1038/s41467-024-48748-6
The research was funded by the MIT Media Lab Consortium and the Brain and Behavior Foundation Research (BBRF) NARSAD Young Investigator Award.
